Brisbane BioTech Vaxxas has snared fresh US government funding to accelerate its needle free vaccination platform for COVID-19 applications, as the company courts private investors.
Vaxxas on Tuesday announced it has been awarded a $3.2 million (US$2 million) funding prize from a US research authority to accelerate its work in collaboration with the University of Queensland.
The win keeps the Brisbane firm in the running alongside US, German and Indian rivals for the $50 million competition’s later stage prizes, potentially up to $28 million more to fund trials.
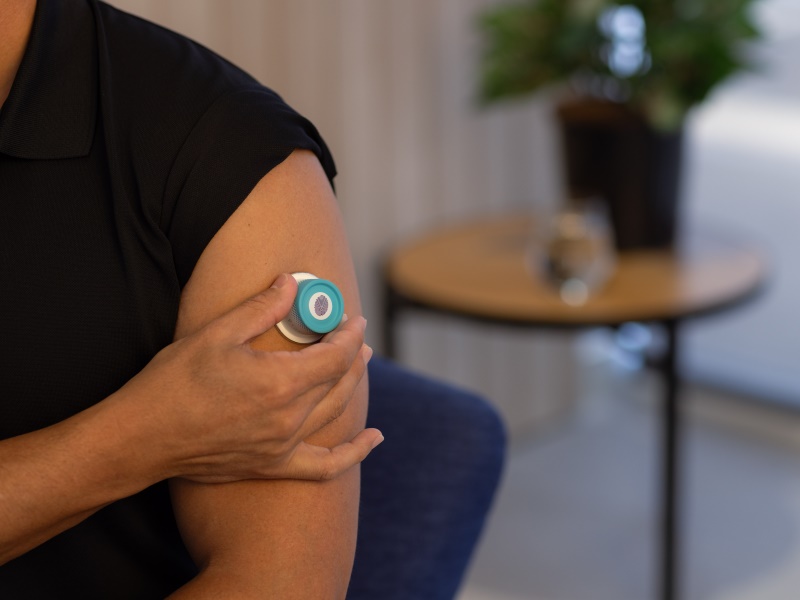
Vaxxas and its rivals are trying to disrupt the 170-year-old method of delivering vaccines with syringes.
The 14-year-old local company says it is on the cusp of commercialising pain free patches that require less vaccine and has fewer supply chain issues than traditional needle or syringe delivery, after completing several human clinical trials.
The core technology was developed in the University of Queensland, and the company was established as a startup in 2011 by the university’s commercialisation group UniQuest.
Early equity backers include Brandon Capital and US-based HealthCare Ventures. The company was reportedly briefing potential new private investors in October as it looks to raise another $100 million privately ahead of a potential IPO later this year or in early 2026.
Vaxxas’ latest boost comes from the US Biomedical Advanced Research and Development Authority and its $50 million Patch Forward competition.
The US competition is specifically aimed at the commercialisation of microarray patch-based RNA vaccines for COVID-19, seasonal influenza and pandemic influenza.
The Australian firm and partner the University of Quensland BASE facility have advanced with three rivals as a concept stage winner and will now vie for down selection for the competition’s larger pre-clinical and clinical trials funding.
“We’re excited about the potential for our technology to play an important role in effectively protecting populations against dangerous respiratory infectious diseases such as influenza and COVID-19,” Vaxxas chief executive David Hoey said.
Do you know more? Contact James Riley via Email.

